The current aim for the muskrat eDNA part of Life-MICA is scaling-up and real-life implementation of the eDNA approach and transference of sample processing to the water laboratories.
In order to compare results between the lab at the University of Amsterdam and the labs at Wetterskip Fryslân and Waterproef (Noord-Holland) we started sampling this year in regions with a higher chance of muskrat presence. For Fryslân this meant sampling near the borders, and for Noord-Holland sampling in a region where they have trapped the most muskrats in the past few years. Both regions have a low population of muskrat compared to other parts of the Netherlands.
Fryslân
In Fryslân a polder that has been designated as empty of muskrat was also sampled to determine if the eDNA results matched the evaluation based on traditional tracking methods (polder empty of muskrat), this did indeed match (Fig 1, region indicated by blue arrow). Figure one shows the results of sampling in Fryslân.
As of 29-06-22, 449 monitoring samples haven been taken in Fryslân, most by boat, as well as 78 localisation tracks, 70 point samples and 9 control samples (taken to confirm no more muskrat are present after catch actions).
As of 22-06-22, 34 muskrats had been trapped in the sampled areas (27 in waterways/tracks with eDNA and 7 in tracks without eDNA, but that were adjacent to tracks with eDNA).
Noord-Holland
In North Holland, there were many more traces of muskrat eDNA in the sampled region than in previously sampled areas. This allowed us to make a good comparison between the laboratories. But the high number of samples containing muskrat eDNA makes this region less suitable for the mainstream eDNA approach. Because of the significant population of muskrat in the area in the period of May/June the trappers focussed on catching the muskrats in this area. Figure 2 shows the catches of the muskrat overlayed on the eDNA results.
As of 22-06-22, 215 muskrat had been caught in the sampled area (182 in waterways/tracks with eDNA and 33 in tracks without eDNA, but again these were adjacent to tracks with eDNA). In Noord-Holland 167 monitoring tracks were sampled, as well as 163 localisation tracks and 181 point samples.
Most tracks in Noord-Holland were sampled by hand, which is more labour intensive and time consuming than sampling by boat. Areas with these population levels are more suited for follow up with eDNA after an intensive trapping effort, to determine if there are remaining muskrats, rather than the method used for monitoring areas that are historically empty or have very low presence.
Coypu
For the Coypu the aim is not so much scaling up but determining how to best integrate the eDNA method in the tracking efforts. Coypu behave differently from muskrat and are also caught alive in cages instead of traps in burrows. This makes certain parts of the field approach less useful for coypu (localisation of burrows). 31 areas were sampled for coypu presence, and as of 22-06-22, there were 23 catches, of which 21 corresponded with eDNA signal.
Wetterskip Fryslân and Waterproef processed all the samples of their respective regions for this year, and are thus capable of routinely processing samples. Transfer of analysis of coypu eDNA samples has been initiated with Aqualysis.
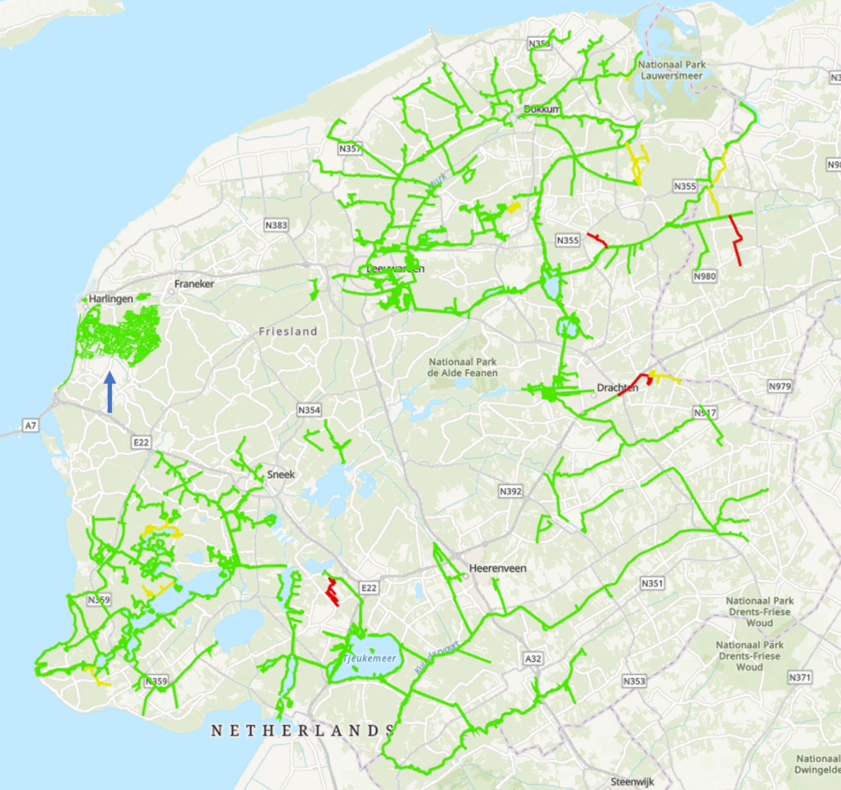 Figure 1. Results monitoring Fryslân. Green: eDNA negative, Yellow: eDNA weakly positive and Red: eDNA positive. Both yellow and red tracks are followed up in the protocol.
Figure 1. Results monitoring Fryslân. Green: eDNA negative, Yellow: eDNA weakly positive and Red: eDNA positive. Both yellow and red tracks are followed up in the protocol.
The blue arrow indicates the polder which was sampled in order to determine if there were muskrat remaining in an area that had been marked as empty by traditional methods. This polder was sampled by hand/quad.
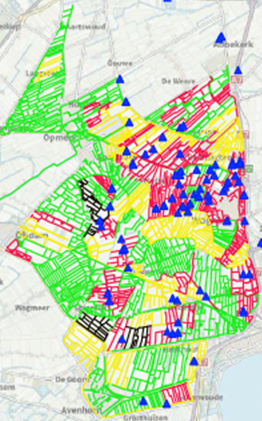 Figure 2. Sampled area Noord-Holland
Figure 2. Sampled area Noord-Holland
Green: eDNA negative, Yellow: eDNA weakly positive and Red: eDNA positive.
Blue triangles: catches
